What a Saponification Laboratory Can Teach Us about Darwin's Enchanted Pond
In science education, one of the functions of the laboratory is to bring to life theoretical principles. Good laboratories enable chemistry students to make inferences as to what is happening at the micro level, based upon macro-level observations1: more specifically, how reactant atoms and molecules interact with one another in product formation. A chemical reaction is evident at the microscopic level when we see with our eyes color changes of solutions, the formation of precipitates, the release of light, heat or smoke, or we smell the emission of an odor.
Imagine a teacher's delight overhearing a student exclaim, "Oh wow, now the class discussions make sense!" while watching several test tubes cloud up when products form by combining clear salt solutions (such as salt water and silver nitrate). For that excited student, the lab exercise served its purpose: theoretical principles became real.
The beauty of chemical reactivity becomes practical for my organic chemistry students whenever they have the opportunity to perform a saponification reaction just in time for the holidays. To a bowl of 800 grams of various oils and fats, they slowly stir in lye solution (105 grams of drain cleaner crystals dissolved in 240 mL of distilled water); with an added touch of peppermint fragrance, they now have … a luxurious handmade soap, like magic!

The process as described above sounds easy enough, but it isn't. Tension builds with a full list of admonitions:
• don't mis-measure your oils
• make sure the fatty acid count exceeds the hydroxide ion count by 10%
• double-check, no… triple-check your lye calculations
• don't mix hot lye in with the melted fats
• add only enough heat to your solid fats to liquefy them—don't damage them with too much heating!, and,
• don't overmix or you'll never get the soap in the mold
Finally, once the engineered soap mixture is poured into molds students are cautioned: "keep it warm or you'll kill the progress of the reaction… but not too warm."
If everything is done just right, each student ends up with enough soap to cut into eight 4-oz bars for gift giving. The three-page protocol they use that I wrote was birthed from countless failed attempts at making soap, by following the recipes and anecdotes of online crafters. My early efforts yielded soaps that would not solidify, were greasy, or developed lye pockets. Through all this, I learned there are innumerable ways to make unusable soap, and really very limited ways to make gift-able soap. Not until I consulted a more authoritative work on scientific soapmaking2 was I able to craft perfect soaps every time, and ultimately share that hard-earned knowledge.
For the holiday soap-making laboratory then, students observe a powerful theoretical: the hallmark of chemical change, written in its most general form as:
oils(l) + fats(s) + lye solution(aq) → soap(s) + glycerin(s).
The properties of the reactants—on the left side of the arrow—differ greatly from that of the products—on the right side. The greasy properties of the fats/ oils, together with the caustic nature of the lye, are exchanged for the mild, cleansing and skin-softening properties of a high-quality soap. For my junior soap-makers then, theoretical concepts surrounding chemical change become real.
Soap molecules (aka fatty acid salts) are a schizophrenic lot: their long hydrophobic tails—made up of just carbon and hydrogen atoms—despise water (being hydrophobic), yet their electrically charged heads—with two oxygen atoms strategically placed—love it (being hydrophilic). This dual nature makes soap the perfect cleaning agent as their hydrophobic tails love to associate with dirt, oil, and one another, thus pushing their charged heads to the outside in the formation of a three-dimensional sphere called a micelle3—with the dirt molecule smack-dab in the middle of the structure4 (below right).
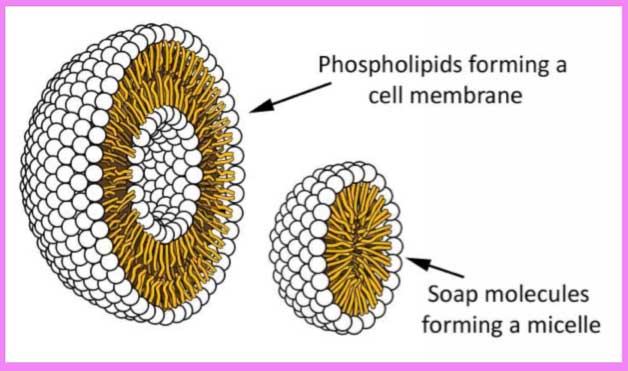
What is of most interest is how soap molecules are functional sisters to the phospholipids making up cell membranes4 (above left), yet not nearly as complex. Phospholipids not only have to get along with one another and their watery environment, but innumerable proteins and sugars as well. Their form and function make it a spectacular and truly magical notion to consider these emerging from the "warm little ponds" Darwin wistfully conceived of.
Darwin's enchanted pond was charged with a chemical engineering feat much greater than my own. For soap-making, I merely had to cut already existing molecules (triglycerides) apart, yet the warm little pond had to synthesize these phospholipids—along with literally countless other molecules—from scratch materials, strategically linking one atom to another.
As I mentioned, good chemistry laboratories provide valuable lessons as macro-level observations inform us as to that which is occurring at the micro level. My soap-making journey taught me that "simple," functional molecules are just plain difficult to make, demanding reaction conditions that are anything but random. For the brave scientist's soul theorizing that intelligent agency was a requisite for the formation of early life, there is nothing like a saponification laboratory to make such a theoretical concept real.
Notes
1. Johnstone, A. H. (2000). Teaching of chemistry-logical or psychological?. Chemistry Education Research and Practice, 1(1), 9-15.
2. Dunn, K. M. (2010). Scientific soapmaking: The chemistry of the cold process. Clavicula press.
3. Karty, J. (2014). Organic Chemistry: Principles and Mechanisms. WW Norton & Company.
4. Image above modified from the original Phospholipids aqueous solution structures.svg, from Wikimedia Commons, by artist Michelle Ruiz Villarreal (2007).
graduated summa cum laude from California State University, Fresno, with a BS in molecular biology and a minor in cognitive psychology. As an undergraduate, she conducted research in immunology, microbiology, behavioral and cognitive psychology, scanning tunneling microscopy and genetics - having published research in the Journal of Experimental Psychology, and projects in scanning tunneling microscopy. Having recently completed an M.Ed. from University of Cincinnati and a Certificate in Apologetics with the Talbot School of Theology at Biola University, Emily is currently an instructional designer/content developer for Moody Bible Institute and teaches organic chemistry and physics. As a former Darwinian evolutionist, Emily now regards the intelligent design arguments more credible than those proffered by Darwinists for explaining the origin of life.
• Get SALVO blog posts in your inbox! Copyright © 2026 Salvo | www.salvomag.com https://salvomag.com/post/raising-the-bar-of-soap


















